Efficiency cookies are applied to know and analyze the key effectiveness indexes of the website which allows in delivering a better person encounter with the website visitors. Analytics Analytics
Analytical cookies are accustomed to know how visitors communicate with the website. These cookies enable deliver information on metrics the volume of website visitors, bounce rate, visitors source, and many others. Advertisement Ad
If your result in is assignable, then have a corrective and preventive motion and history exactly the same in acceptable structure.
Personnel assigned to complete actions over the media runs needs to be adequately educated to the prerequisite while in the media operate protocol and also the duties to be performed.
All cartoned provides are decontaminated during the anteroom place by removing them from transport cartons and wiping or spraying having a disinfecting agent, for example sterile IPA, though staying transferred to some cleanse, sanitized cart or other conveyance for introduction to the buffer or clean up location.
Microbiology and environmental monitoring personnel have already been adequately educated and capable for the treatments stated earlier mentioned, and penned documentation of this coaching is available and recent.
Action Limitations / Treatment in the Case of Failed Simulations:- Steps for analysing the reason for contamination and an investigation thereafter should be founded. On exceeding the motion Restrict, a requalification is immediately demanded. According to ISO 13408-128 an investigation needs to be carried out in the event of exceeding the warning Restrict (one contaminated unit approximately ten, 250 units) and the operate needs to be repeated. If the warning Restrict is exceeded once again, it indicates the media fill has failed and the entire Main qualification must be recurring (three consecutive runs of media fill needs to be successful). In the situation of requalification (usually just about every 6 months one particular effective media fill) exceeding of the warning Restrict in two consecutive operates has to be evaluated as exceeding the motion Restrict.
PROCESSING A created description of precise coaching and functionality analysis application for individuals associated with the usage of aseptic approaches for that preparation of sterile solutions needs to be formulated for each site. This plan equips the personnel with click here the appropriate knowledge and trains them from the required skills important to conduct the assigned responsibilities.
Inspection shall be finished by certified microbiologists or staff trained by qualified microbiologists to recognize contaminated media filled containers.
Immediately after leak test, transfer the goods vials from the thoroughly clean plastic crates horizontally within the cassette from a single over the opposite, lot wise independently.
Environmental Checking Along with the analysis and verification of personnel aseptic approaches and of the adequacy of compounding processes and techniques (see Staff Training and Analysis in Aseptic Manipulation Abilities area), evaluation and verification of your adequacy of the sterile compounding setting is crucial, especially for getting ready substantial-possibility preparations. Analysis of environmental excellent is done by measuring equally the overall range of particles and the volume of practical microorganisms during the managed air environments with the compounding space. Certification that every LAFW and barrier isolator is operating appropriately and fulfills the air excellent requirement of ISO Course 5 (refer to Clean Rooms and Barrier Isolators and Table one during the Environmental Quality and Control segment) is done by a certified operator(s) making use of existing, condition-of-the-art Digital air here sampling at least each 6 months and Every time the LAFW or barrier isolator is relocated.
Good quality and Operations administration shall be notified in one organization day of confirmation of constructive units.
Test executed to exhibit that media will support microbial progress, as needed by Pharmacopeia that specifies problem organisms, inoculum level, and incubation conditions
Microbiological Environmental checking must be carried out to cover the complete media fill method for manufacturing region by Settle plate, Active Air sampling, Swab test and personnel checking as per the latest SOP.
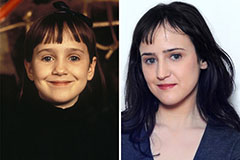 Mara Wilson Then & Now!
Mara Wilson Then & Now! Mr. T Then & Now!
Mr. T Then & Now!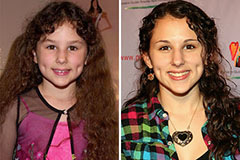 Hallie Eisenberg Then & Now!
Hallie Eisenberg Then & Now! Michelle Trachtenberg Then & Now!
Michelle Trachtenberg Then & Now! Atticus Shaffer Then & Now!
Atticus Shaffer Then & Now!